The U.S. Food and Drug Administration has approved Phathom Pharmaceuticals' Voquezna (vonoprazan), a novel potassium-competitive acid blocker, as a new treatment for adults for with all grades of erosive esophagitis or erosive gastroesophageal reflux disease (GERD).
retrieved 8 November 2023 from https://medicalxpress.com/news/2023-11-fda-voquezna-erosive-esophagitis-gerd.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.Oct 18, 2023Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use ourThank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.Get weekly and/or daily updates delivered to your inbox.
Belgique Dernières Nouvelles, Belgique Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Antireflux mucosal ablation safe, effective for refractory reflux diseaseAntireflux mucosal ablation (ARMA) cuts short-term gastroesophageal reflux disease (GERD) symptoms in two-thirds of patients undergoing the endoscopic treatment, according to a study published in the Journal of Gastroenterology and Hepatology.
Antireflux mucosal ablation safe, effective for refractory reflux diseaseAntireflux mucosal ablation (ARMA) cuts short-term gastroesophageal reflux disease (GERD) symptoms in two-thirds of patients undergoing the endoscopic treatment, according to a study published in the Journal of Gastroenterology and Hepatology.
Lire la suite »
 Increase expected in approved AI-based medical imaging productsThe number of U.S. Food and Drug Administration (FDA)-approved artificial intelligence (AI) products is expected to increase from 69 in 2022 to 350 in 2035, according to a study published online Oct. 16 in the Journal of the American College of Radiology.
Increase expected in approved AI-based medical imaging productsThe number of U.S. Food and Drug Administration (FDA)-approved artificial intelligence (AI) products is expected to increase from 69 in 2022 to 350 in 2035, according to a study published online Oct. 16 in the Journal of the American College of Radiology.
Lire la suite »
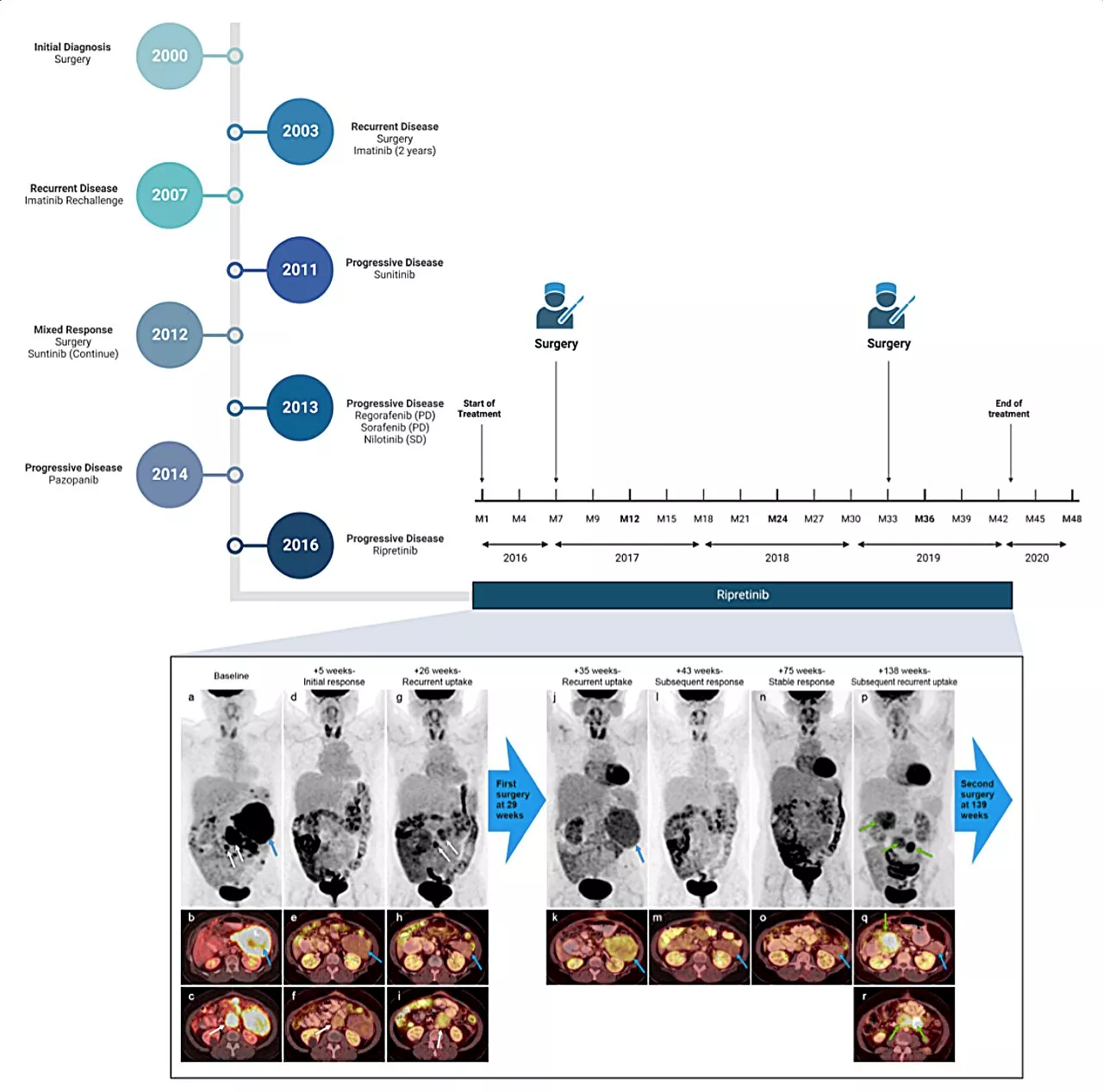 Management of recurrent gastrointestinal cancer with ripretinib and surgeryRipretinib is a tyrosine kinase inhibitor that was approved by the United States FDA in 2020 for treatment of advanced gastrointestinal stromal tumor (GIST) in patients who received prior treatment with three or more tyrosine kinase inhibitors.
Management of recurrent gastrointestinal cancer with ripretinib and surgeryRipretinib is a tyrosine kinase inhibitor that was approved by the United States FDA in 2020 for treatment of advanced gastrointestinal stromal tumor (GIST) in patients who received prior treatment with three or more tyrosine kinase inhibitors.
Lire la suite »
 Policing Board approves selection of Northern Ireland’s next chief constableThe police oversight body in Northern Ireland has approved the selection of the region’s next chief constable, with the decision now passed to the Government for sign-off.
Policing Board approves selection of Northern Ireland’s next chief constableThe police oversight body in Northern Ireland has approved the selection of the region’s next chief constable, with the decision now passed to the Government for sign-off.
Lire la suite »
 NHS approves preventative breast cancer drug that halves risk of disease in ‘major step forward’ for...A daily pill that could cut the risk of developing breast cancer by half is to be offered to 300,000 at-risk women.
NHS approves preventative breast cancer drug that halves risk of disease in ‘major step forward’ for...A daily pill that could cut the risk of developing breast cancer by half is to be offered to 300,000 at-risk women.
Lire la suite »
